Walking Trees, Parasitic Flowers, and Other Remarkable Plants: An Illustrated Guide

The French botanist and biologist Francis Hallé has spent 40 years in pursuit of the strange and beautiful plant specimens of the rainforest. In his “Atlas of Poetic Botany,” excerpted below, he invites readers to tour the farthest reaches of the rainforest in search of exotic — and poetic — plant life.

Like any good tour guide, Hallé tells stories to illustrate his facts. Readers learn about, among other things, Queen Victoria’s rubber tree; legends of the moabi tree (for example, that powder from the bark confers invisibility); a flower that absorbs energy from a tree; plants that imitate other plants; a tree that rains; and a fern that clones itself.
Hallé’s Atlas aims to show that “the equatorial forest isn’t the ‘green inferno’ that colonialists and adventurers have so often confronted,” as he writes in the book’s introduction, but “a universe with magical allure.” His drawings represent an investment in time that returns a dividend of wonder more satisfying than the ephemeral thrill afforded by the photograph.
Hevea brasiliensis (A. Juss.) Muell. Arg.
EUPHORBIACEAE
Seringueira, Rubber tree
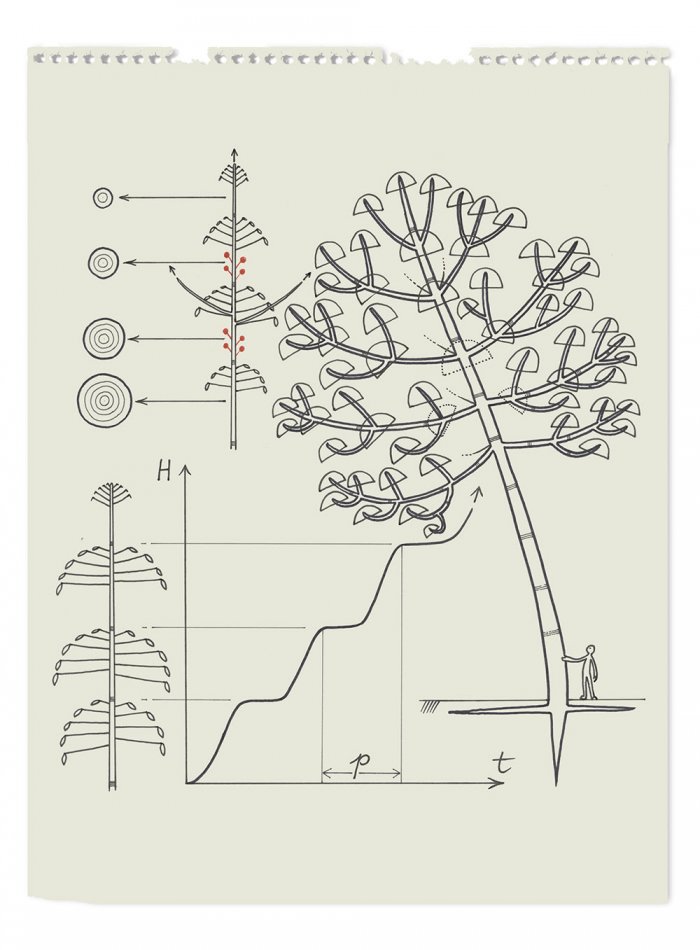
It’s a very beautiful tree. One of the biggest in the Amazon, it can reach 60 meters in height. Its trunk is splendid, but always scarred with grooves: Euphorbiaceae contain latex, the white milk from which rubber is made.
If this tree intrigues me, it is because of its unusual rhythm of development, in particular. Hevea grows, then stops and loses its leaves before starting to grow again. The pattern isn’t annual, but occurs over a period of 42 days. Why does the rubber tree grow this way, as if it were governed by seasons — like European trees, but below the equator? It remains a mystery. Interrupt this rhythm, and the tree won’t make latex. And if all the latex is emptied out, the tree dies. No one know what purpose the latex actually serves for the plant itself.
People primarily take interest in plants for what they can give them. For instance, medicinal plants are used without anybody asking why it is that they synthesize precious, valuable molecules in the first place. Everything has a function in the animal world; nothing there is without a purpose. The sheer number, among plants, of examples such as the rubber tree — whose latex has no clear reason for existing — makes me think they don’t work like animals. Are they capable of selfless acts? The hypothesis isn’t very satisfying, but that might be another thing that sets them apart from animals.
Europeans have known about latex ever since America was discovered: Christopher Colombus mentions it in his account of his travels. Rubber was brought to Europe for the first time in the 18th century by Charles Marie de La Condamine and François Fresneau de La Gataudière. The French naturalists were struck by wonder when they saw Amerindians playing with a ball that bounced when dropped. La Condamine authored the first scientific description of the material, whose name in Quechua is caotchu (cao, “wood,” and tchu, “which weeps”).
In Brazil, the name for the rubber tree is seringua, and the man who harvests latex a seringueiro. Every night, the seringueiro leaves his hut and follows the same path: a circle through the forest. He bleeds each hevea along the way and leaves behind a coconut shell or a can to collect the latex. The tree’s no hemophiliac, so it scars in a couple of hours.
At around eight in the morning, the seringueiro comes back home but almost immediately sets out again on the same route, this time carrying a bag into which he empties the latex that’s collected in the interim. Once the second round is done, he needs to hurry and make a fire with palm fruit, the smoke of which helps shape the latex into large globes.
I’ve had occasion to admire the ingenuity displayed by seringueiros — and by Amerindians, for that matter — in finding practical use for rubber. For instance: Somewhere in the Amazon, a man on a bike gets a flat. Undaunted, he removes the inner tube, looks for the right tree — needless to say, there’s one around — and makes a cut in the bark. The latex oozes out, and he puts a little on the leak. The cyclist has just enough time for a cigarette before the patch is done; a few quick pumps, and the bike’s back in operation.
Alternatively, a seringueiro might need a pair of boots. He fashions the shape of feet, right and left, with pieces of wood. Each day, he puts the molds into a latex bath, then lets them dry. A few days later, he’s got a pair of boots!
But why do seringueiros always follow the same path in the forest? Why not grow a plantation of trees? Herein lies the great drama of rubber in Brazil: Cultivating hevea is impossible because of a parasite — a fungus called Hemileia vastatrix. To avoid an epidemic, a distance of at least 300 meters between trees is required. Under these conditions, intensive planting won’t work. Henry Ford, the American industrialist, tried anyway in the 1920s. He had a whole worker’s colony — christened “Fordlandia” — built on the banks of the Tapajós river. The thousands of trees that were planted soon perished because of the fungus and today, Fordlandia’s nothing more than a ghost town. Even a great captain of industry is no match for a mushroom.
The rubber tree claimed victory when it emerged from Brazil without its parasite. Brazilians affirm that the plant was stolen from them. They accuse the Englishman Henry A. Wickham of harvesting 70,000 seeds with the help of Amerindian confederates, tricking custom officials, and bringing the spoils to the director of the Royal Botanic Gardens, Kew, in London. There, it’s said, the seeds were planted in tropical greenhouses, and the trees that grew were carried off to Ceylan (Sri Lanka).
But the story isn’t at all true! Queen Victoria personally, and officially, asked for hevea seeds for Kew Gardens. Honored by the request, the Brazilians obliged. The first seeds didn’t survive the long voyage by sail, as they only live for three weeks. It took a steamboat for new ones to reach England in a sufficiently good state to sprout in hothouses there. The rubber trees obtained in this manner then made their way to Malaysia, where plantations flourished — as they did in Ceylan and throughout Southeast Asia. Thailand is still the main producer of natural rubber.
Brazilians have forgotten the whole story and complain about botanical fraud. The development is understandable: It’s painful for the native land of hevea to have to import natural rubber, and at considerable expense.
Still, the globalization of trade all but guarantees that the parasitic fungus Hemileia vastatrix will find its way to Southeast Asia. Cultivators are getting ready: Recent plantings of hevea are no longer monocultures; instead, care is taken to follow the forest model and put at least 300 meters between trees.
Today, even though most car tires are made out of synthetic rubber, natural rubber is preferred for high-performance products — tires for Formula One racers and airplanes, surgical gloves, and condoms…. On a philosophical level, hevea illustrates that what’s natural will always win out over anything that’s merely artificial.

Helosis cayennensis (Sw.) Spreng.
BALANOPHORACEAE
In 1962, when I was going up La Mana River in French Guiana, I noticed what, at the time, I took to be a red mushroom with a cap that hadn’t opened yet; the next morning, it was dead. Over the next few days, I saw many others, which all died just as quickly. Little by little, I realized that these mushrooms had all stopped living without giving me the chance to see them open up — as if something had happened to them during the night.
I decided to stay awake and watch, and I observed some very curious things over the course of that night. When darkness falls, the caps of these pseudo-mushrooms, which are composed of close-set scales, bring forth… flowers: Little by little, the scales all fall away and a round cluster blooms. As the night progresses, these flowers wither and vanish, leaving a bare, red surface riddled with holes. Toward the end of the night, new flowers shoot out of the holes, and by four in the morning, the top is covered with little white blossoms that smell nice and attract insects. When day breaks, this second generation of flowers is already faded, and everything rots and falls apart.
What I’d taken for a mushroom was in fact a plant with parasitic flowers. Living in the shady undergrowth, Helosis cayennensis has adapted to the absence of light and, therefore, photosynthesis: It has no leaves or chlorophyll. Instead, it extends its roots into those of neighboring trees and draws energy from them, in the form of sugars and other products made by photosynthesis. Over the course of the night, it first brings forth female flowers, and then male ones, which are the ones that smell nice. At dawn, the plant collapses. I was fascinated: I did not know that a plant with flowers could look like a mushroom. I made a great deal of color drawings.
Helosis cayennensis inspires me to reflect on the practice of botany. Most of my colleagues dream of discovering a new plant. I myself don’t care about finding a new plant that needs a new name. That’s not my job. What interests me is the biology. My dream is to do away with the need for names and classifications and bring botany back to the study of the biology of plants.
To get back to Helosis cayennensis: It performs a legitimate form of parasitism, which, all in all, is perfectly moral. In tropical forests, the big trees catch the greatest part of the light up in the canopy. So down at ground level, the small plants — which are unable to employ photosynthesis in the shade — take their energy where they can find it: in the roots of the trees. It’s only fair.

Codariocalyx motorius (Houtt.) Ohashi
FABACEAE PAPILIONOIDEAE
My first voyage to China dates to 1989. The Centre de coopération internationale en recherche agronomique pour le développement (CIRAD) in Montpellier asked me evaluate the botanical garden of Xishuangbanna in the southernmost part of the country, right next to the border with Laos. The garden had been founded during the Mao era, when Europeans weren’t welcome.
I arrived on a Sunday morning, and right away, the director, Chen Jin, asked me: “Do you know the dancing plant?” He took me to some plants growing in pots, little shrubs that didn’t really seem too impressive. Their leaves, which look like those of green beans, are gray. Although they have pretty, pink flowers, these plants appear unremarkable on the whole — modest and retiring. But Chinese people were sitting all around the pots, clapping their hands and shouting. “They’re making the plant dance,” Chen Jin explained. Indeed, the two lateral folioles of each leaf moved when sound was made.
“Could you sing a French song to see if that works too?” they asked me. A Breton sea shanty and… it worked! To the immense delight of onlookers, the plant started dancing.
Before making the trip, I’d been told: “Watch out. The Chinese are intensely nationalistic — they don’t want anybody pilfering their seeds.” All the same, I couldn’t help eyeing the fruits that covered the “dancing plants” — a profusion of little gray pods, like green beans. I eventually submitted a timid request: Would the garden deign to give me a few seeds? Without further ado, the Chinese got up, went off looking for a big bag, and helped me collect some.
I took them back to France, where the plant can develop quite well in a greenhouse. The botanical garden at Les Cèdres, Saint-Jean-Cap-Ferrat, was the first place I grew one. The researchers here wondered if movement occurred in response to the current of air produced by clapping or singing, so they put a transistor next to the plant. It danced without any problem.
Codariocalyx motorius makes it abundantly clear that plants intrigue us even more when they display animal characteristics and offer points of resemblance with ourselves. At the garden in Saint-Jean-Cap-Ferrat, the greenhouse featuring carnivorous plants is full.
What benefit does the plant derive from the astonishing ability to move its leaves when there’s noise? It’s still a mystery! If anything, I’d assume the opposite: that it would want to escape being noticed by predators by not moving at all. Since my visit to China in 1989, knowledge about Codariocalyx motoriush as progressed, and researchers have brought to light a phenomenon I find quite amusing and surprising. When a specimen is left to grow for six months without any noise and efforts are made to make it dance, it will move a little, but very slowly. But it is trained every day, it will start dancing better and better, like a ballerina practicing. The plant needs training, in the athletic sense of the word, which is necessarily based on some kind of memory.
We are far from solving the riddle of why this plant moves and how it does so. Needless to say, there is a reason: It involves a complex mechanism that implies receptors for sound waves. No plant would just set up a mechanism like that, but we are still looking for what it is. For all that, Codariocalyxhas been known for quite a long time: Charles Darwin himself, in “The Power of Movement in Plants” (1880), sang its praises, and was quite aware that this plant moves. Science is often held to represent a cumulative process, but alas, this isn’t the case: Discoveries are made, which the next generation then forgets. Unfortunately, it happens all the time in botany.
At any rate, one thing stands firm about Codariocalyx: This plant senses sound, even though it doesn’t have any ears.
Rhizophora mucronata Lam.
RHIZOPHORACEAE
Mangrove
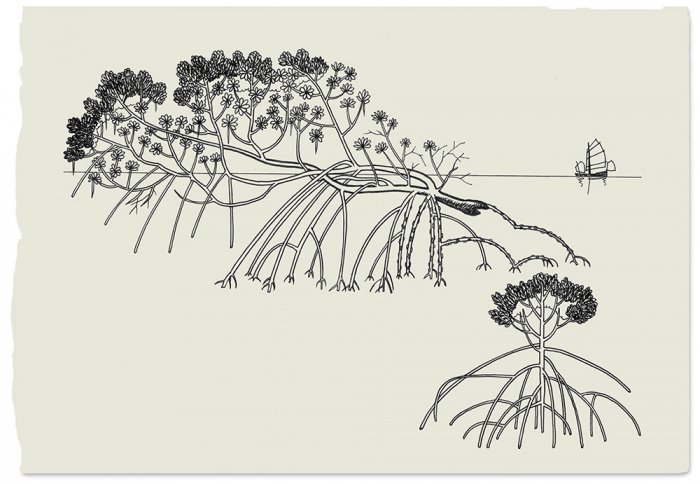
Mangrove forests are the simplest and best-known tropical forests between land and sea, since few trees can handle the difficulties posed by saltwater and daily tides. The grayish silhouettes of mangroves, repeated ad infinitum, dot the landscape of low coasts in the tropics, where they perch on prop roots growing in the fine silt. The trunk and branches grow vertically, without discernible rhythm; since its feet are planted in the seawater, the mangrove doesn’t experience a dry season, so its growth doesn’t follow any pattern. To thrive in this soft, unstable soil, which lacks oxygen and is submerged at high tide, it has developed ingenious strategies: its leaves have the ability to get rid of the salt absorbed, and its aerial stilts give its roots room to breathe.
One finds two kinds of mangrove in a forest. There are those with a trunk that rises up from the soil; these specimens are sprung from a seed and don’t move. Then there are mangroves whose trunk flattens out at the base; these trees stem from a branch at a low elevation — and they walk!
To understand what is going on, we have to look at how these trees start out. A mangrove seed sprouts and brings forth a little plant. As it grows, prop roots appear, which enable it to breathe and occupy a stable position on the mud; it’s like a little tree on stilts. As the mangrove develops, more and more prop roots descend from its branches. At some point, they break off because they are no longer being fed by the trunk at all; now, the branches feed directly from their own roots. This is known as vegetative propagation by marcottage. The most striking thing is that, once detached from the trunk, the branches walk. Arial photos taken at one-year intervals show that the branches move four or five meters a year. The bottom branch, which is always moving, heads for the sea, and when it can no longer reach the bottom — when it loses its footing — it stops.
Is this tree really walking? When we walk, our physical mass moves as a whole. But no displacement of matter occurs with mangroves; the way they grow just makes it look that way. The “movement” is the growing process, four to five meters a year. The branch experiences necrosis and vanishes at one end, while it keeps developing on the other.
Mangroves form amphibious forests that are often quite dense, and create a natural barrier between the sea and the land. In tropical countries affected by cyclones, tempests, and hurricanes, these forests are quite useful: they reduce the damage caused by winds and waves, even when a tsunami strikes. Mangroves also protect the coastal area from the erosion caused by swell and marine currents; they filter pollutants and heavy metals in the water; like all the world’s plants, they trap carbon dioxide; and finally, they provide a habitat for important marine fauna. Countries that have destroyed their mangroves for their tannins or to make firewood regret their actions. Luckily, this kind of tropical forest is easy to plant and grows rapidly.

Rafflesia arnoldii R. Br.
RAFFLESIACEAE
Bunga patma in Malaysia
We were walking in the Sumatran forest when, all of a sudden, a horrible smell overwhelmed us — like clogged toilets, or a strike by garbage collectors in the middle of August. This stench makes botanists laugh; for me, it calls to mind memories of the Asian forest. It means we’re close to a Rafflesia flower, a parasitic plant discovered and described in 1818 by the English botanist Joseph Arnold who named it in homage to Sir Thomas Raffles.
To find it, one must follow the path described by the pestilential odor. Then, there’s no missing it. It’s the biggest flower in the world: up to a meter across, 30 centimeters high, and weighing some seven kilograms. Needless to say, Rafflesia doesn’t care about being discreet: It has five petals, thick and tough, and a crown of stamens and pistils the color of rotten meat. Given this smell and color, you can be sure that flies pollinate Rafflesi; there’s always a cloud of them.
The flower is the only part of the plant that’s visible. Its stalk and roots are inside the parasite’s host: Tetrastigma, which belongs to the vine family (Vitaceae). The distance between the two plants can reach several meters; even so, Rafflesiadraws the water and nutrients it needs from the liana to which it’s attached.
In 2013, Rafflesia arnoldii caused a minor sensation. At the San Diego Botanic Garden, one of them bloomed in a greenhouse. The staff made it do so by planting a Tetrastigma, then waiting until it was big enough to hold the weight of the flower. When the time was right, they made a cut in the vine and put Rafflesia seeds next to it. Rafflesia is a riddling plant. It rains a great deal in Sumatra, so how does the flower — which is shaped like a basin — manage to avoid overflowing with water?
Francis Hallé is a botanist and biologist who specializes in tropical rainforests and tree architecture. He is Professor Emeritus at the University of Montpellier. Hallé is the author of “Atlas of Poetic Botany,” from which this article is excerpted.